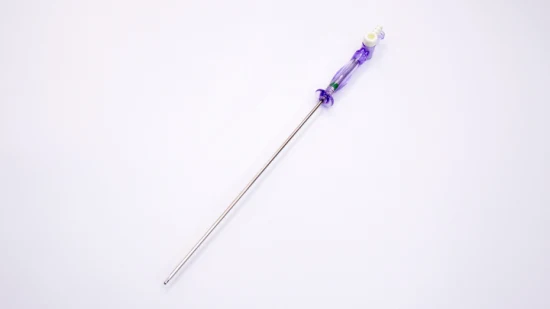
FDA 510K Disposable Medical Veress Needle for Laparoscopy Insufflation 120mm
Basic Info
| Model NO. | VN-120 |
| Working Length | 120mm |
| Ventilation Capacity | 3L |
| Custmization | Yes |
| Transport Package | Peel Pouch Package |
| Specification | ISO13485, CE |
| Trademark | TK |
| Origin | China |
| HS Code | 9018321 |
| Production Capacity | 500, 000 PCS/Year |
Product Description
GTK Veress Needles Insufflation Needles Pneumoneedle Laparoscopic Instruments China factoryCompany Profile
GTK Medical is a hi-tech medical devices manufacturer of more than 17 years R&D and manufacturing expertise. GTK veress needles are exported to more than 40 countries and used in more than 1000 hospitals worldwide.


G T.K veress needles have been exported to 40 countries, most of which are European and American countries, such as the USA, UK, France, Germany, Italy, Belgium, Spain, Japan, South Korea.
Detailed Photos
| Veress Needles Specification | |
| Product Code | Working Length (mm) |
| VN-120 | 120 |
| VN-150 | 150 |
| VN-120L | 120 |
| VN-150L | 150 |
Certifications

1. Strong Innovative Capability:GTK Medical has applied more than 600 patents at home and abroad, including more than 200 granted patents.2. Excellent quality management system:
The quality management system of T.K Medical passed FDA Factory registration ( FDA Factory registration number is 3008191256), ISO 13485 and CE Marking.
| Q: Are you a factory or trading company? |
| A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience. |
| Q: What is the core strength of GTK Medical? |
| A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products. |
| Q: What makes GTK Veress Needle stand out? |
| A: Double warning system confirms safe insertion. Ergonomic handle ensures for convenient and easy insertion. |
| Q: Do you provide free samples of GTK Veress Needle for clinical trial use? |
| A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation. |
| Q: Do you have exported experiences to large medical devices company/companies? |
| A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe. |
| Q: Do you accept factory audit prior to formal partnership? |
| A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021. |
Please do not hesitate to contact me, I am always here waiting for assisting you!






